It’s true that titanium dioxide does not rank as high for UVA protection as zinc oxide, it ends up being a small difference (think about it like being 10 years old versus 10 years and 3 months old). This is not easily understood in terms of other factors affecting how sunscreen actives perform (such as the base formula), so many, including some dermatologists, assume that zinc oxide is superior to titanium dioxide for UVA protection. When carefully formulated, titanium dioxide provides excellent UVA protection. Its UVA protection peak is lower than that of zinc oxide, but both continue to provide protection throughout the UVA range for the same amount of time.
. It actively seeks to reduce its carbon footprint and engages in initiatives that foster biodiversity and support local ecosystems.
Les pouvoirs couvrant et éclaircissant du lithopone normal sont supérieurs à ceux de la céruse et de l'oxyde de zinc, mais inférieurs au Dioxyde de Titane pur, étant le meilleur blanc sous tout rapport. C'est le sulfure de zinc qui, avec son indice de réfraction de 2,37, est l’élément opaque ; le 2nd composé, le sulfate de baryum, joue un rôle de diluant minéral et favorise l'efficacité de la diffusion du premier.
Titanium dioxide is produced in two main forms. The primary form, comprising over 98 percent of total production, is pigment grade titanium dioxide. The pigmentary form makes use of titanium dioxide’s excellent light-scattering properties in applications that require white opacity and brightness. The other form in which titanium dioxide is produced is as an ultrafine (nanomaterial) product. This form is selected when different properties, such as transparency and maximum ultraviolet light absorption, are required, such as in cosmetic sunscreens.
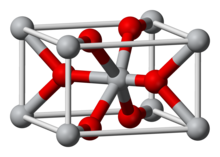
The aim of this work was to examine particularly the Degussa P25 titanium dioxide nanoparticles (P25TiO2NPs) because they are among the most employed ones in cosmetics. In fact, all kinds of titanium dioxide nanoparticles (TiO2NPs) have gained widespread commercialization over recent decades. This white pigment (TiO2NPs) is used in a broad range of applications, including food, personal care products (toothpaste, lotions, sunscreens, face creams), drugs, plastics, ceramics, and paints. The original source is abundant in Earth as a chemically inert amphoteric oxide, which is thermally stable, corrosion-resistant, and water-insoluble. This oxide is found in three different forms: rutile (the most stable and substantial form), brookite (rhombohedral), and anatase (tetragonal as rutile), of these, both rutile and anatase are of significant commercial importance in a wide range of applications [3]. Additionally, the nano-sized oxide exhibits interesting physical properties, one of them is the ability to act as semiconducting material under UV exposure. In fact, TiO2NPs are the most well-known and useful photocatalytic material, because of their relatively low price and photo-stability [4]. Although, this photoactivity could also cause undesired molecular damage in biological tissues and needs to be urgently assessed, due to their worldwide use. However, not all nanosized titanium dioxide have the same behavior. In 2007, Rampaul A and Parkin I questioned: “whether the anatase/rutile crystal form of titanium dioxide with an organosilane or dimethicone coat, a common titania type identified in sunscreens, is appropriate to use in sunscreen lotions” [5]. They also suggested that with further study, other types of functionalized titanium dioxide could potentially be safer alternatives. Later, Damiani found that the anatase form of TiO2NPs was the more photoactive one, and stated that it should be avoided for sunscreen formulations, in agreement with Barker and Branch (2008) [6,7].
Lithopone was discovered in the 1870s by DuPont. It was manufactured by Krebs Pigments and Chemical Company and other companies. The material came in different seals, which varied in the content of zinc sulfide. Gold seal and Bronze seals contain 40-50% zinc sulfide, offering more hiding power and strength. Although its popularity peaked around 1920, approximately 223,352 tons were produced in 1990. It is mainly used in paints, putty, and in plastics.



 These minerals can improve the tear strength, abrasion resistance, and flexibility of rubber materials These minerals can improve the tear strength, abrasion resistance, and flexibility of rubber materials
These minerals can improve the tear strength, abrasion resistance, and flexibility of rubber materials These minerals can improve the tear strength, abrasion resistance, and flexibility of rubber materials The facility operates under a strict code of conduct that prioritizes ecological preservation and community welfare The facility operates under a strict code of conduct that prioritizes ecological preservation and community welfare
The facility operates under a strict code of conduct that prioritizes ecological preservation and community welfare The facility operates under a strict code of conduct that prioritizes ecological preservation and community welfare